
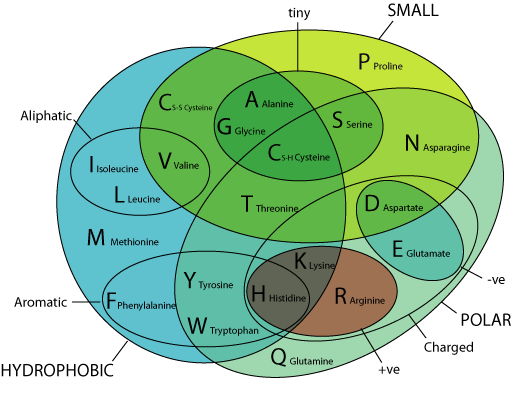
A single conservative replacement, such as replacing Ala with Gly or adding polar amino acids to the N- or C-terminus may improve solubility. At a physiological pH, Asp, Glu, Lys and Arg will contain charged side chains. TIP – Keep hydrophobic amino acid content below 50% of the total sequence length and to include at least one charged amino acid for every five amino acids. Uncharged (polar): Asn, Cys, Gly, Gln, Pro, Ser, Thr, Tyr Hydrophobic (non-polar): Ala, Ile, Leu, Met, Phe, Trp, Val Inclusion or exclusion of hydrophobic or hydrophilic amino acids in a peptide sequence will impact the ability to synthesize, purify and solubilize the final peptide material in aqueous solutions.

Peptide SolubilityĪmino acids are classified according to a hydropathy index, based on the hydrophobic or hydrophilic properties of their side chains.
Increased length equates to increased number of amino acid couplings, which may result in solubility issues during purification when attempting to remove synthesis impurities. Give special attention to sequences greater than 30 amino acids in length. Peptide purity typically decreases as the sequence length increases. These considerations include sequence length, solubility and sequence stability. When selecting peptides for custom synthesis, several important factors should be considered during the design process.


 0 kommentar(er)
0 kommentar(er)
